Kiln Types & Parts
Kiln Structure
Shape—square/rectangular, cylinder/hexagon
Type of top arch - catenary, Roman/sprung arch, dome
Kiln Building Materials
Refractory brick: 1) soft insulating, 2) hard. Soft brick is lightweight and can be cut easily with a hand saw, but is not acceptable for salt or woodfire kilns. Hard brick is heavy and useful for salt of woodfire kilns, and for lining fireboxes. Both are rated for heat tolerance as K23 (2300°F), K26, K28, etc.
Fiber: “Kaowool” or other brands of flexible refractory blanket looks similar to thick cotton quilt batting. It makes a very light weight insulating layer, can be fastened into a light wire framework for a top hat type kiln. Caution: after firing it becomes brittle and it is harmful to breath the dust created when it is moved.
Other: earth as in hillside kilns, shallow pits.
Kiln Room or Yard Requirements
Ventilation— All kiln rooms must have adequate fresh air intake and a good exhaust system to carry away heat, carbon monoxide, and other metal fumes. This is important for both gas and electric kilns. Do NOT sit by a kiln, even if the room is vented. Carbon monoxide poisoning makes you feel drowsy and unable to save yourself.
Safety— always check with local fire codes, and comply with them. Have licensed professionals install the gas or electric service, and have it inspected by local officials. Make sure that your insurance coverage is adequate and up to date. Many potters have lost everything due to carelessness.
Glaze Firing Atmosphere - Oxidation or Reduction
Story: How the Potter Fooled Flame into Making Pretty Colors
The burners are flaming nicely; there's plenty of oxygen wafting through the kiln, and all oxidizing well in the kiln room. Then Potter walks in, nudges the damper closed a little, and "Oh no, she's at it again." says Flame. All of a sudden he is starving for oxygen. All Flame wants to do is burn, but no, his draft has been cut down and now he must go searching through the ware to find his next meal. He's so thin he's making carbon monoxide. "Ah," Flame says, "there's some iron and copper in that pot over there. I'll sneak a little oxygen from them. Mmmm, that's tasty, I hope Potter doesn't notice the colors shift. But of course the potter noticed. She shoved the damper in, didn't she? That fool, Flame, fell for it again, and Potter got her beautiful stoneware celadon, flambe, and tenmoku glaze colors. Tomorrow she'll trick him into making metallic luster in the raku firing.
Oxidation Atmosphere - Normally an oxidation atmosphere, or complete combustion, is desired for efficient heating. An electric kiln has an oxidation atmosphere unless combustible material is present in the clay.
Why a Reduction Atmosphere is Desirable - Reduction of iron and copper (and some other oxides) create beautiful colors in certain cases. Excess heated carbon (monoxide) steals oxygen atoms from any iron or copper present. This changes the molecular structure of both. Carbon monoxide becomes carbon dioxide, and the number of atoms present in the copper and iron effects the network of interlinked atoms that reflects light, thus changing their color.
Draft Control - Air for combustion is controlled in various ways
Pit/primitive Firing: Low temperature firing can be done in a shallow pit in the ground, or a simple/primitive kiln made of a garbage can with holes cut in the side for air, or a simple stack of common or fire brick. Pit fires are usually fueled by wood and/or dried cow chips, and are often used to create a “reduction atmosphere” (see Reduction, below)
Garbage Can Firing: fueled by sawdust packed around the ware and lit from the top to slowly burn down.
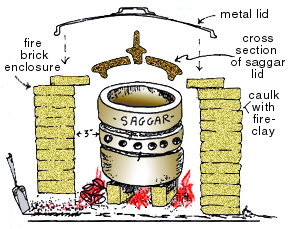
Saggar Firing: A saggar is traditionally a ceramic boxlike container used to either
a) enclose and protect ware from flying ash inside a large kiln chamber during a wood firing, or
b) to contain organic material around a pot (such as straw soaked in salt brine) to produce a reduction atmosphere inside the saggar (Example: Bizen ware). Aluminum foil can be used as a “saggar” for firings up to around 1650 degrees Farenheit.
Brick Kilns
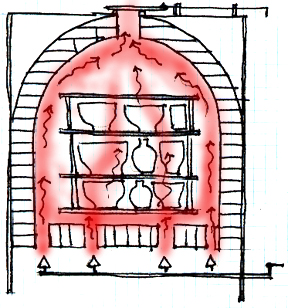
Updraft—exit port and damper to control draft is at the top of the chamber.
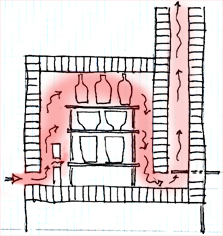
Downdraft—exit port is at the bottom of the inner chamber; the damper to control draft is in the chimney, on the side.
Crossdraft—burners set up so that flames cross.
Muffle—heat is channeled through tubes or by bag walls, to protect the ware a direct hit from the flames.
Loading the Chamber with ware
Load ware from the front, top, or "top hat" (kiln chamber raised off base by pulley)
Bisque and Glaze firings are stacked differently. Work will move as it shrinks in firing. Pieces can touch or be stacked on each other carefully in a bisque firing, but usually not in the glaze firing. Allow enough space around ware for even distribution of heat toward the center of the stack.
Monitoring the Firing - by color, pyrometer, pyrometric cones, and oxygen analyzer
1. The color changes from cold darkness to red orange, orange, yellow, to white hot. Experienced "firemen" in the old days could tell by color what the temperature was. However, we now wear welding goggles to protect our eyes from ultra-violet light, and this makes it difficult to accurately see the color during a firing.
2. The pyrometer is a high-temperature thermometer that measures the heat in the kiln, but not the effect that the heat is having on the clay and glazes (the heatwork).
3. Pyrometric cones (or witness cones) are made of unfired ceramic raw materials that are formulated to melt at a certain temperature, provided that the heat has risen at a specified rate. They are a more accurate indication of the heatwork that has taken place in the ware being fired. Normally 3 consecutive cones are placed in a pack at the correct angle - a lower warning cone, the cone desired, and an "oops, it went too high" cone. When the desired cone has bent halfway over, it is time to turn off and close up the kiln.
4. An oxygen analyzer monitors the atmosphere in the kiln so that optimal oxidation and reduction atmospheres may be created when desired.
Heatwork = Time + Temperature
Chemical and physical changes are necessary to transform clay into a hard ceramic material. This requires heating for a period of time, hence the term heat and work done, or heatwork. [Source: The Potters Dictionary of Materials and Techniques (1975) by Frank Hamer]
Heat is the sun's energy which is stored in fuel. It is liberated by fire, or oxidation. Heat is excited particles which vibrate and re-arrange within the clay resulting in a hard material that will no longer dissolve into a lump in water. Exothermic - heat releasing. Endothermic - heat absorbing.
Burning fuel is an exothermic process, i.e. it releases energy (leaving ash in the case of wood), while the process of clay hardening in heat is an endothermic process, in that it absorbs the heat energy that was freed by the burning fuel. (Clay does have an exothermic reaction in its cooling stage when the glazes crystallize and give off some heat energy.
Sample Firing Time - cone 06-04 Bisque
Up to 400 @50-80 degrees per hour = 4-6 hours
400-1200 @150 degrees per hour = 5.5 hours
1200-1900 @200 degrees per hour = 4 hours to cone 06-04 bisque
total 12-18 hours to heat + 12-18 hours to cool